How To Find Polarity Of A Molecule
1.7: Polarity of Molecules
- Page ID
- 30254
Dipole moments occur when there is a separation of charge. They can occur between ii ions in an ionic bond or between atoms in a covalent bail; dipole moments arise from differences in electronegativity. The larger the difference in electronegativity, the larger the dipole moment. The distance between the accuse separation is also a deciding factor into the size of the dipole moment. The dipole moment is a measure of the polarity of the molecule.
Introduction
When atoms in a molecule share electrons unequally, they create what is called a dipole moment. This occurs when one cantlet is more electronegative than some other, resulting in that atom pulling more than tightly on the shared pair of electrons, or when one atom has a lonely pair of electrons and the departure of electronegativity vector points in the aforementioned manner. One of the most mutual examples is the water molecule, fabricated upwards of one oxygen atom and two hydrogen atoms. The differences in electronegativity and alone electrons give oxygen a partial negative charge and each hydrogen a fractional positive charge.
Dipole Moment
When two electrical charges, of contrary sign and equal magnitude, are separated by a altitude, a dipole is established. The size of a dipole is measured by its dipole moment ((\mu\)). Dipole moment is measured in debye units, which is equal to the distance between the charges multiplied by the accuse ( 1 debye equals iii.34 x 10 -30 coulomb-meters). The equation to figure out the dipole moment of a molecule is given beneath:
where
-
μ is the dipole moment, -
q is the magnitude of the accuse, and -
r is the distance between the charges.
The dipole moment acts in the direction of the vector quantity. An case of a polar molecule is
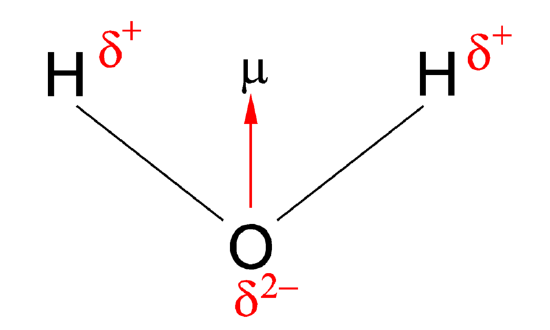
Figure 1: Dipole moment of water
The vector points from positive to negative, on both the molecular (net) dipole moment and the individual bond dipoles. The tabular array above shows the electronegativity of some of the mutual elements. The larger the difference in electronegativity between the two atoms, the more electronegative that bond is. To be considered a polar bond, the difference in electronegativity must exist large. The dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together.
| Instance one: Water |
|---|
| The h2o molecule moving picture from Figure 1 tin can be used to make up one's mind the direction and magnitude of the dipole moment. From the electronegativities of water and hydrogen, the divergence is 1.2 for each of the hydrogen-oxygen bonds. Next, considering the oxygen is the more electronegative cantlet, it exerts a greater pull on the shared electrons; it also has two lone pairs of electrons. From this, it tin can be concluded that the dipole moment points from betwixt the 2 hydrogen atoms toward the oxygen cantlet. Using the equation above, the dipole moment is calculated to be 1.85 D by multiplying the distance betwixt the oxygen and hydrogen atoms by the charge difference between them and then finding the components of each that betoken in the management of the internet dipole moment (remember the angle of the molecule is 104.5˚). The bond moment of O-H bond =one.5 D, and so the cyberspace dipole moment =two(1.5)×cos(104.5/2)=1.84 D. |
Dipole Moments
It is relatively like shooting fish in a barrel to measure dipole moments. Identify substance between charged plates--polar molecules increase the charge stored on plates and the dipole moment tin can be obtained (has to practise with capacitance). Polar molecules align themselves:
- in an electric field
- with respect to i another
- with respect to ions
Nonpolar
Figure 2: Polar molecules marshal themselves in an electric field (left), with respect to one some other (middle), and with respect to ions (correct)
When proton & electron shut together, the dipole moment (degree of polarity) decreases. Nonetheless, equally proton & electron get further apart, the dipole moment increases. In this case, the dipole moment calculated as:
The debye characterizes size of dipole moment. When a proton & electron 100 pm apart, the dipole moment is
- When proton & electron are separated past 120 pm,
- When proton & electron are separated by 150 pm,
- When proton & electron are separated by 200 pm,
Polarity and Structure of Molecules
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might non accept whatever overall polarity, depending upon its shape. The simple definition of whether a circuitous molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers prevarication at the same point in space, so the molecule has no overall polarity (and is non polar).
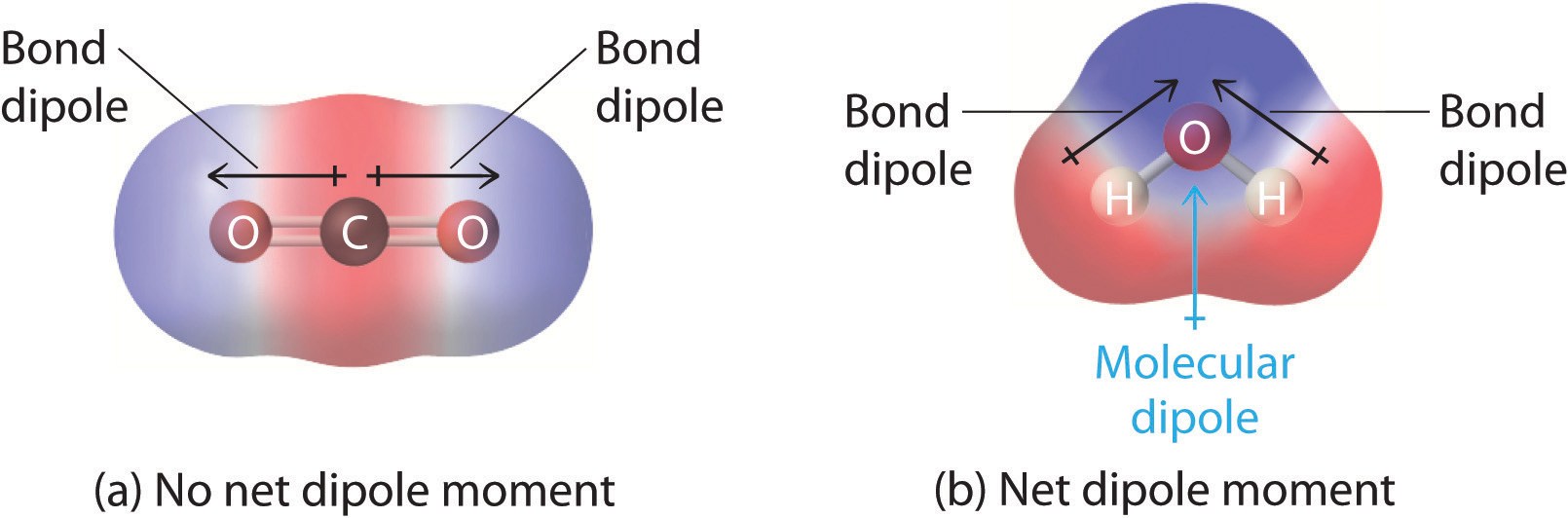
Effigy three: Charge distrubtions
If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. A molecule can merely be polar if the structure of that molecule is not symmetric.
A good example of a nonpolar molecule that contains polar bonds is carbon dioxide. This is a linear molecule and the C=O bonds are, in fact, polar. The fundamental carbon will accept a net positive charge, and the two outer oxygens a cyberspace negative charge. However, since the molecule is linear, these two bond dipoles cancel each other out (i.e. vector addition of the dipoles equals null). And the overall molecule has no dipole (
Although a polar bond is a prerequisite for a molecule to have a dipole, non all molecules with polar bonds exhibit dipoles
Geometric Considerations
For
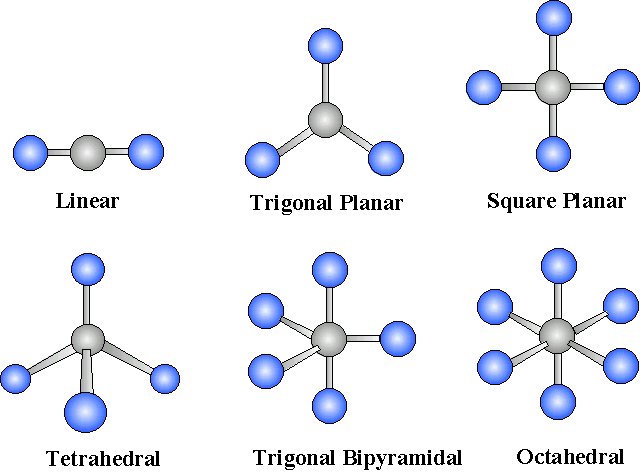
Effigy 4: Molecular geometries with verbal cancelation of polar bonding to generate a non-polar molecule (
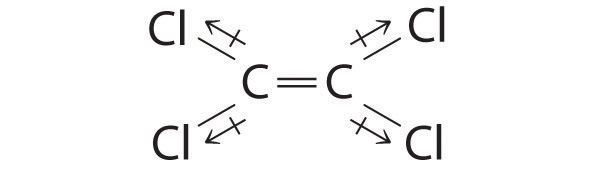
| Case 2: |
|---|
| Although the C–Cl bonds are rather polar, the individual bond dipoles cancel one another in this symmetrical construction, and CltwoC=CCl2 does not take a net dipole moment. |
| Case 3: |
|---|
| C-Cl, the central polar bond, is 178 pm. Measurement reveals 1.87 D. From this information, % ionic grapheme can exist computed. If this bond were 100% ionic (based on proton & electron), |
| Example 4: |
|---|
| Since measurement 1.87 D, % ionic = (1.vii/8.54)x100 = 22% u = one.03 D (measured) H-Cl bond length 127 pm If 100% ionic, ionic = (ane.03/vi.09)x100 = 17% |
| Compound | Bail Length (Å) | Electronegativity Difference | Dipole Moment (D) |
| HF | 0.92 | i.ix | one.82 |
| HCl | 1.27 | 0.9 | ane.08 |
| HBr | 1.41 | 0.7 | 0.82 |
| How-do-you-do | 1.61 | 0.iv | 0.44 |
Although the bail length is increasing , the dipole is decreasing as you move down the halogen group. The electronegativity decreases as we move down the grouping. Thus, the greater influence is the electronegativity of the ii atoms (which influences the charge at the ends of the dipole).
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_01%3A_Structure_and_Bonding/1.12%3A_Polarity_of_Molecules
Posted by: campnottake.blogspot.com

0 Response to "How To Find Polarity Of A Molecule"
Post a Comment